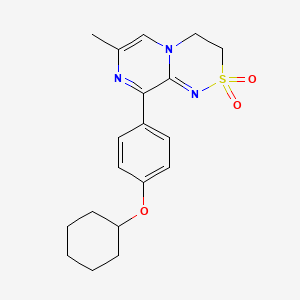
TAK-653 – (1358751-06-0)
Osavampator, also known by its developmental code names TAK-653 and NBI-1065845, is an investigational drug being studied for its potential in treating treatment-resistant depression. Developed by Takeda Pharmaceuticals, osavampator is a selective positive allosteric modulator (PAM) of the AMPA receptor. It enhances the effects of glutamate at the AMPA receptor by slowing the receptor’s desensitization and internalization, thereby potentially influencing AMPA receptor-mediated transcription more directly.
Research indicates that osavampator may offer a novel approach to treating depression by targeting the AMPA receptor, which is believed to be crucial in the antidepressant effects of certain NMDA receptor antagonists like ketamine. Unlike ketamine, osavampator does not induce hyperlocomotion in rats, and initial human trials suggest it has less pronounced CNS stimulatory properties, with no severe adverse effects reported.
Osavampator’s potential as a non-psychotomimetic antidepressant is a key reason for its investigation. It provides a significant safety margin against convulsions compared to therapeutic doses in rats, and its minimal direct AMPA agonist properties suggest a lower risk of seizures and neurotoxicity. This profile makes osavampator a promising candidate for clinical applications, particularly in patients who do not respond to traditional antidepressants.
The above information is displayed for information purpose only, and has not been reviewed by EON nor does EON attests or validates the accuracy nor does it constitutes a recommendation or validation.
https://en.wikipedia.org/wiki/Osavampator
| Other Names | Osavampator |
|---|---|
| IUPAC Name | 9-(4-cyclohexyloxyphenyl)-7-methyl-3, 4-dihydropyrazino[2, 1-c][1, 2, 4]thiadiazine 2, 2-dioxide |
| CAS | 1358751-06-0 |
| Molecular Weight | 373.5 |
| Molecular Formula | C19H23N3O3S |
| SMILES | CC1=CN2CCS(=O)(=O)N=C2C(=N1)C3=CC=C(C=C3)OC4CCCCC4 |